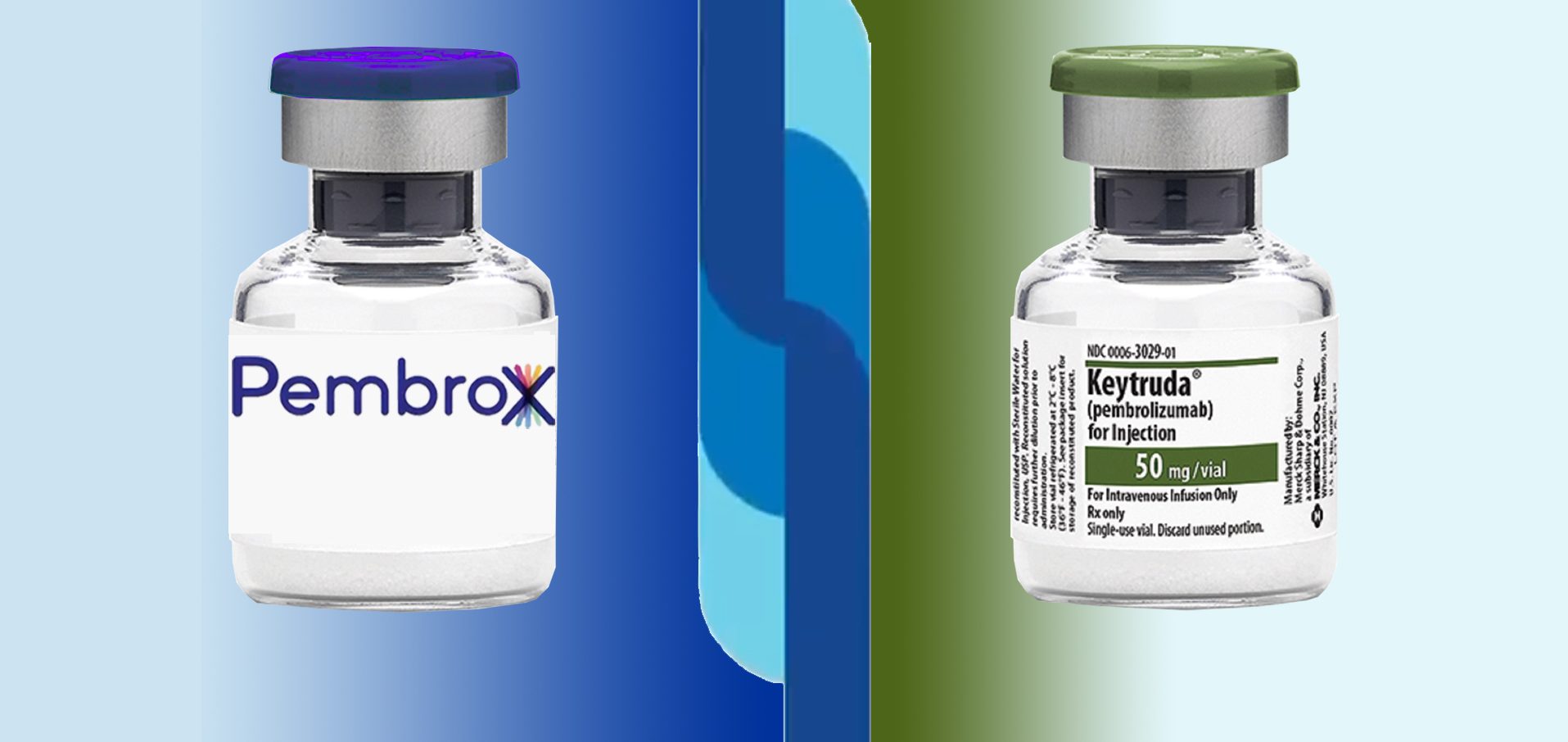
Elea is shaking up the market again with another local version of a blockbuster drug. Last year, it launched the «Argentine Ozempic» in the form of the oral semaglutide, Dutide. Now it’s launching PembroX, a biosimilar of Merck & Co’s oncology blockbuster Keytruda.
Argentina is again making headlines due to analogues and biosimilars of international blockbusters. The drugmaker Elea has launched a biosimilar of Keytruda, the star monoclonal anybody of the US multinational Merck & Co (known as MSD outside of North America).
Based on pembrolizumab, the new product has around 20 indications and will compete in the Argentine market with imports of MSD’s blockbuster, which was first approved by the FDA in September 2024. According to the national vademecum, Keytruda is priced at AR$17.1 million (US$ 16,039) per 100 mg vial while Elea’s biosimilar has launched at a price of AR$16.8 million (US$ 15,718). The real battlefield will be public tenders. MSD won a AR$10.87 billion (US$ 10.4 million) contract in 2024. See list of Keytruda indications | See article on the tender in Spanish
This oncologic drug has been on Elea’s radar for some time. In an interview with La Fábrica Podcast, Hugo Sigman, one of the group’s shareholders, said he believed biologics had exorbitant prices due to a lack of price control in the industry, with the market for speciality products representing 5% of global units and 30% of turnover. He mentioned Keytruda as «the best-known case«, noting its sales of US$ 30 billion. «I think there is an issue there that is putting the public-private financing of governments, as well as patient access, in crisis,» he said. See article in Spanish
According to half-year results published in July, sales of Keytruda grew by 22% in constant currency to US$ 14.22 billion in that period. MSD said the growth was due to continued strong global momentum in metastatic indications, including certain types of non-small cell lung cancer, renal cell carcinoma, squamous cell carcinoma of the head and neck and triple-negative breast cancer, among others.
Elea is no stranger to seizing an opportunity to offer an alternative to a big-name product. Last year, it launched its own version of semaglutide, the wildly successful GLP-1 drug used in Novo Nordisk’s Ozempic and Wegovy to treat type 2 diabetes and obesity. It launched Dutide as an oral version more convenient than injections. Novo’s own oral version, Rybelsus, was approved by the FDA in 2019 but hasn’t been brought to Argentina. See article on the approval of Dutide in Spanish
US$1 = AR$1,069 (BCRA rate Tuesday 07/01/2025)